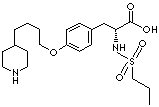
PRODUCT IDENTIFICATION
151065-53-1 (HCl)
150915-40-5 (HCl hydrate)
TOXICITY
H.S. CODE
CLASSIFICATION
Cardiovascular agent, Fibrin modulating agent, Fibrinolytic agent, Hematologic agen, Platelet aggregation inhibitor, Sulfonamide, Piperidine
EXTRA NOTES
Treatment of unstable angina.
Platelet glycoprotein-IIb/IIIa receptor antagonist.
PHYSICAL AND CHEMICAL PROPERTIES
white to off-white crystalline powder
135 - 137 C
REFRACTIVE INDEX
NFPA RATINGS
AUTOIGNITION
FLASH POINT
EXTERNAL LINKS & GENERAL DESCRIPTION
Drug Information Portal (U.S. National Library of Medicine) - Tirofiban
http://www.nejm.org/doi/
A Comparison of Aspirin plus Tirofiban with Aspirin plus Heparin for
Unstable Angina
http://circ.ahajournals.org/
Tirofiban, a tyrosine derivative with a molecular weight of 495 kD, is a novel,
highly selective inhibitor of fibrinogen binding to platelet GP IIb/IIIa. Preclinical and clinical studies have confirmed
that tirofiban inhibits ex vivo platelet aggregation in response to a variety of
agonists, including ADP, collagen, epinephrine, and thrombin. Potential
advantages of such a drug include immediate onset of action, rapid reversal of
antiplatelet activity after drug discontinuation, suitability for multiple
repeat administrations, and high specificity for the IIb/IIIa receptor.
http://www.drugbank.ca/
Mechanism of action:
Tirofiban is a reversible antagonist of fibrinogen binding to the GP IIb/IIIa
receptor, the major platelet surface receptor involved in platelet aggregation.
Platelet aggregation inhibition is reversible following cessation of the
infusion of tirofiban.
APPEARANCE
white to off-white crystalline powder
IDENTIFICATION
passes Test
ASSAY
99.0% min
134 - 138 C
-13° ~ -16°
IMPURITY
Individual impurity: 0.5% max
Total impurity: 1.0% max
SULPHATED ASH
0.1% max